Laboratory Medicine
Test catalogue
| Factor V Leiden | |
|---|---|
Test NameFactor V Leiden |
|
|
|
Specimen HandlingCollection Instructions: Must indicate anticoagulant status to avoid delay. Approval from the Medical Director is REQUIRED for inpatient samples. Referred-in patients can be tested without approval from the Medical Director. Outpatients can be tested from pre-approved clinics and ordering physicians. Write your initials, date and time of collection on the collection label. Transportation: Send sample at room temperature to the Core lab as soon as possible. Sample Rejection Criteria: Clotted sample, grossly hemolyzed samples, unlabeled or samples with insufficient quantity will be rejected. |
|
Required Documentation |
|
Turnaround Time (TAT)14 days |
Test Utilization |
Reference ValueSee Interpretive Comment. |
|
Test CodeFVL |
MethodologyPCR |
Testing LocationSMH-Molecular Diagnostic |
Other informationLast Updated: June 06, 2019
|
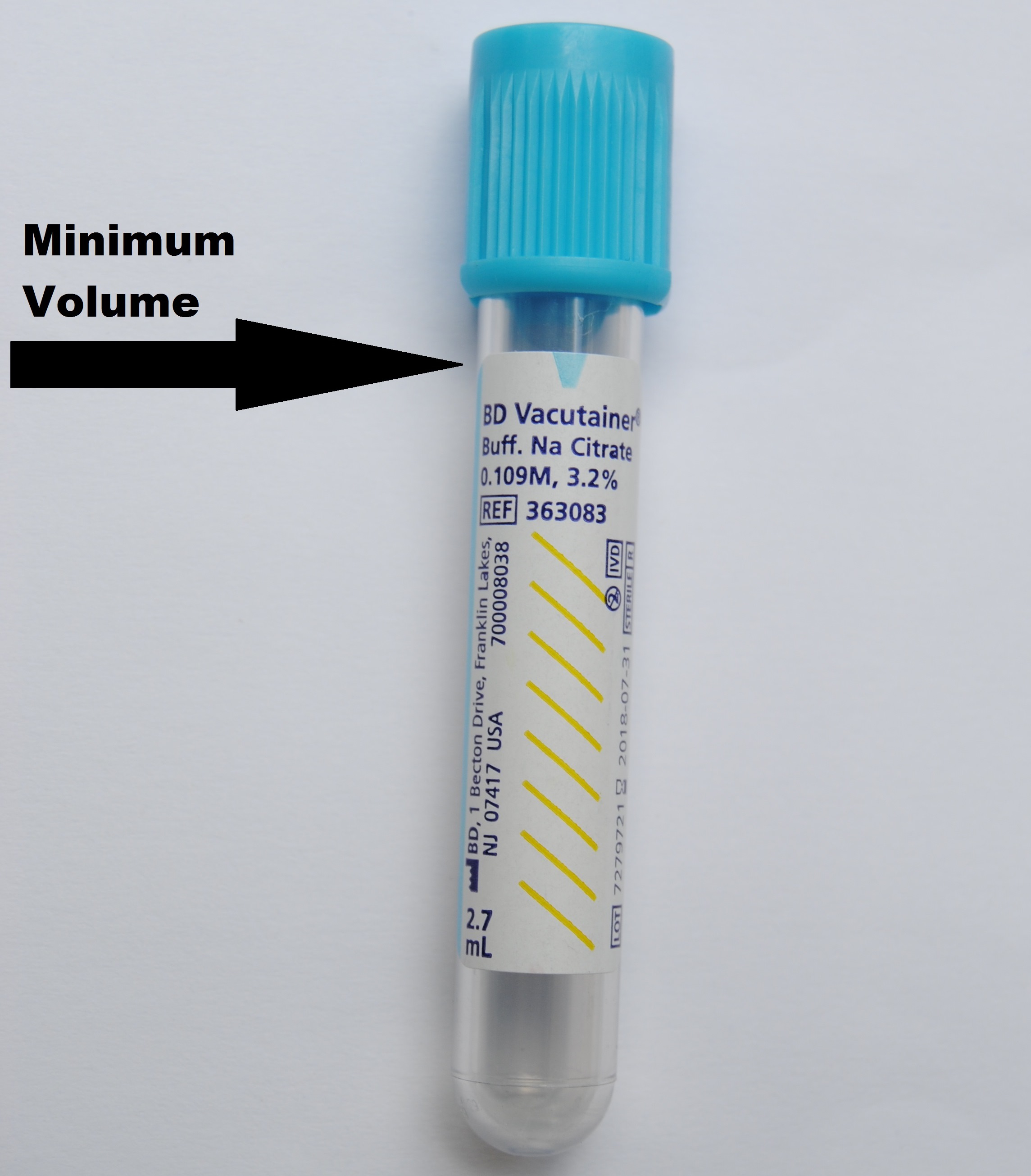 Specimen Type / Requirements
Specimen Type / Requirements