Laboratory Medicine
Test catalogue
| Factor VIII Inhibitor (Human) | |
|---|---|
Test NameFactor VIII Inhibitor (Human) |
|
|
|
Specimen HandlingCollection Instructions: Must indicate anticoagulant status to avoid delay. For inpatient/outpatient samples, refer to the Nijmegen Bethesda Factor VIII Inhibitor Test. Referred-in patients can be tested without a Hematology consult. Write your initials, date and time of collection on the collection label. Transportation: In/Outpatients: Send whole citrated blood at room temperature as soon as possible. Referred-in: Send frozen citrated plasma on dry ice. All samples must be sent to the Core Laboratory. Stability: Specimens are stable for 4 hours post collection at room temperature. Specimens which are not tested within 4 hours can be double-spun to remove the citrated plasma. The citrated plasma is stable at -20°C for up to two weeks or -70°C for up to six months. Sample Rejection Criteria: Clotted sample, grossly hemolyzed samples, unlabeled or samples with insufficient quantity will be rejected. |
|
Required Documentation |
|
Turnaround Time (TAT)Routine: 1 day 4 hours if requested through Haematology consult |
Test Utilization |
Reference ValueNone Detected |
|
Test CodeIN8H |
MethodologyBethesda Assay |
Testing LocationSMH-Special Coagulation |
Other informationLast Updated: June 06, 2019
|
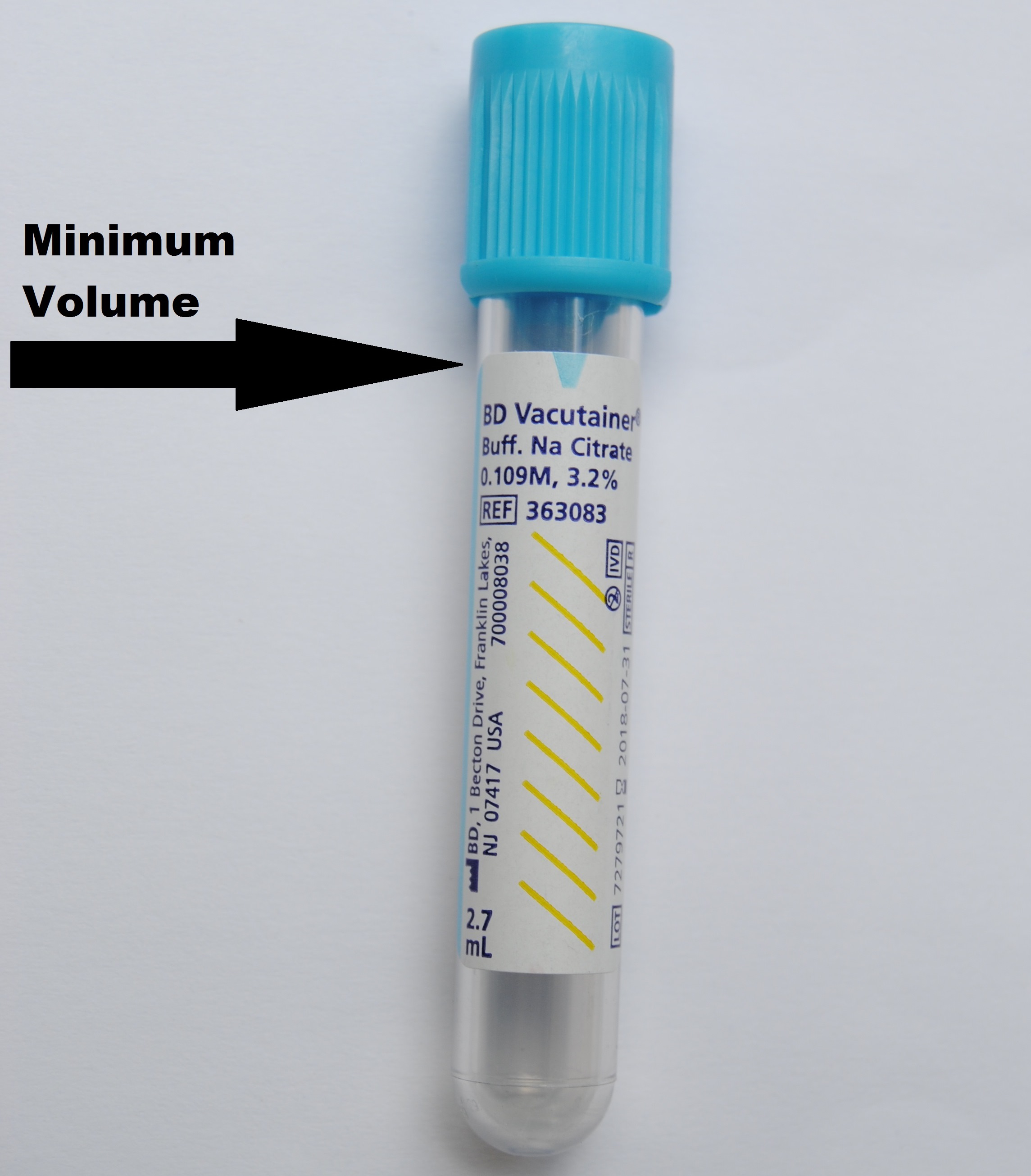 Specimen Type / Requirements
Specimen Type / Requirements